Matrix gel is a soluble basement membrane preparation extracted from EHS mouse tumors rich in extracellular matrix proteins. Its main components include laminin, type IV collagen, heparan sulfate proteoglycans (HSPGs), nidogen, and growth factors such as TGF-beta, EGF, IGF, FGF, tissue plasminogen activator, and other growth factors naturally present in EHS tumors.
Matrix gel was first discovered and described in detail by J. Engelbreth-Holm and Richard Swarm in the mid-1980s in Denmark. The main production process involves extracting/washing the tumor homogenate with saline to obtain soluble proteins, followed by solvent extraction of insoluble complexes from the product, and dialysis with a buffer solution at low temperatures to obtain a colorless to pale yellow solution.

Table: Composition of Matrix Gel [1]
Matrix gel provides support, tensile strength, and scaffold support for tissues and cells. It also serves as a three-dimensional substructure for cell adhesion and movement, as well as a reservoir for growth factors, chemokines, and cytokines. Additionally, it can act as a signal for cell morphology changes and differentiation.
Arcegel matrix gel developed and produced by Arcegen Biotechnology is free from LDEV (Lactate Dehydrogenase-Elevating Virus), with an ultra-low endotoxin content. It has undergone thorough mycoplasma testing to ensure no mycoplasma contamination, including different types of matrix gel such as basic concentration, high concentration, low growth factor, etc.
Application directions

Product features
High Safety: Free from LDEV (Lactate Dehydrogenase-Elevating Virus)
Diverse Concentration: Concentration ranges between 8~20 mg/ml
Good Batch Stability: Strict production quality inspection process ensures stable performance between batches
Low Endotoxin: Endotoxin content ≤ 4 EU/ml
Contaminant Detection: Tested free from mycoplasma, bacteria, and fungal residues
High Single Batch Yield: Single batch yield is above the liter level
Good Compatibility: Compatible with any type of cell culture medium
Migration and Invasion Assay
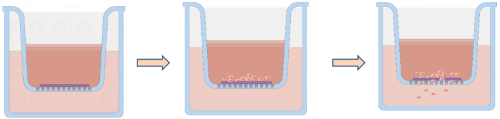
Image: Process of Migration and Invasion Assay
1. Matrix Gel Coating
First, thaw the received Arcegel matrix gel and dilute it in serum-free culture medium at a ratio of 1:8 (final concentration should not exceed 3 mg/ml) to coat the upper chamber membrane of the Transwell insert. Place at 37°C for 30 minutes to allow the Arcegel matrix gel to polymerize into a gel. Hydrate the membrane before use.
2. Preparation of Cell Suspension
1. Starve cells for 12-24 hours to remove the influence of serum (optional).
2. Digest cells, centrifuge after digestion, discard the culture medium, and wash with PBS 1-2 times. Resuspend cells in serum-free culture medium containing BSA to adjust the cell density to 4×104~2×105 cells/ml.
3. Cell Seeding
1. Add 100μl of cell suspension to the Transwell insert.
2. Add 600μl of culture medium containing 20% FBS to the lower chamber of the 24-well plate. Avoid bubble formation between the lower chamber medium and the insert, as bubbles can weaken or even eliminate the chemotactic effect of the lower chamber medium.
3. Cultivate cells: standard culture for 12-48 hours (time mainly depends on the invasive ability of cancer cells).
4. Result Collection
1. Remove the Transwell insert, discard the medium in the well, wash twice with calcium-free PBS, fix with 4% paraformaldehyde for 30 minutes, and air-dry the insert appropriately.
2. Stain with 0.1% crystal violet for 20 minutes, gently wipe off the non-migrated cells on the upper surface, wash with PBS three times, observe and count cells in five random fields under a microscope at 400x magnification.

Figure: Results of crystal violet staining after cell invasion.
FAQ
Q1: What is the reason for the color change (from pale yellow to dark red) in the received matrix?
A: For matrix gel containing phenol red, the main reason for the color change is due to the interaction of phenol red and bicarbonate with CO2. However, the color difference will decrease after equilibration with 5% CO2. After thawing, gently shake the reagent bottle to ensure uniform dispersion of the matrix gel.
Q2: What precautions should be taken when handling matrix gel?
A: All operations should be carried out in a sterile environment, and pre-cooled pipettes should be used to ensure that the matrix gel is in a uniform slurry state.
Q3: How to aliquot, freeze, and use matrix gel?
A: After thawing, Arcegel matrix gel can be aliquoted into multiple small tubes. All aliquoting should be done using pre-cooled cryovials. Quickly freeze and store to avoid multiple freeze-thaw cycles. All items involved in the process should be pre-cooled before use, and pre-cooled pipettes, tips, and tubes should be used to handle the matrix gel.
Related products
|
Product Types |
Product Name |
Catalog Number |
Specifications |
|
Basic Concentration |
C231001 |
5/10 mL |
|
|
C231002 |
5/10 mL |
||
|
Low Growth Factor |
C231003 |
5/10 mL |
|
|
C231004 |
5/10 mL |
||
|
High Concentration |
C231005 |
5/10 mL |
|
|
Arcegel Matrix High Concentration, Phenol Red-Free, LDEV-Free |
C231006 |
5/10 mL |
|
|
C231007 |
5/10 mL |
||
|
Specialized for Stem Cells |
C231008 |
5/10 mL |
|
|
Specialized for Organoids |
Arcegel Matrix for Organoid culture, Phenol Red-Free, LDEV-Free |
C231009 |
5/10 mL |